Abstract
Background
Clonal cytopenia of undetermined significance (CCUS) is a risk factor for development of myeloid neoplasms (MN) such as myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). Therapy-related myeloid neoplasm (t-MN) is defined as the development of a MN in the context of prior DNA damaging therapies including chemotherapy, radiation, and immunosuppressive therapies. CCUS diagnosed in the setting of prior DNA damaging therapy (t-CCUS) and its outcomes are not known. The aim of this study was to study clinicopathological features and outcome of t-CCUS and compare it with t-MN.
Methods
Patients who had received DNA-damaging therapy and subsequently determined to have CCUS or t-MN were identified. The diagnosis of CCUS was made if the patient had non-diagnostic bone marrow biopsy evaluation combined with evidence of pathogenic myeloid somatic genetic alterations using conventional cytogenetics or next generation sequencing (NGS) panel. Pathogenic/likely pathogenic variants (PV) were stratified by DTA (DNMT3A, TET2, and ASXL1) genes vs. others. Event-free survival (EFS) was calculated using interval from t-CCUS to t-MN with death as a competing risk. Overall survival (OS) for t-CCUS and t-MN patients was calculated using interval from t-CCUS or t-MN diagnoses respectively to the last follow up. Statistical analysis was performed using JMP (v14.1, SAS Institute).
Results
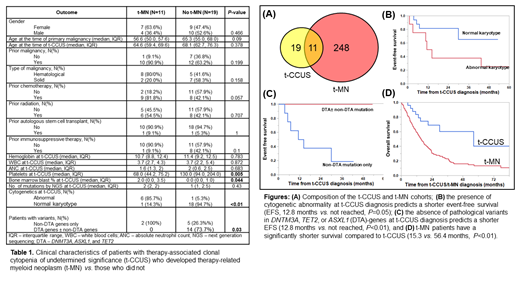
We identified 30 patients with t-CCUS and 259 patients with t-MN. Eleven (37%) t-CCUS patients developed t-MN and were included in both cohorts (Fig. A). Clinical characteristics of t-CCUS patients are shown in Table 1. Briefly, age (median, interquartile range or IQR) at the diagnosis of primary malignancy and t-CCUS was not different between those who developed t-MN and those did not (P=0.09 and 0.378 respectively). There was no difference in hemoglobin, white blood cells, or ANC between the two groups. Patients who developed t-MN had a significantly lower platelet count (68 vs. 130, P<0.01) and higher bone marrow blast percent (2 vs. 0, P=0.04) compared to those who did not.
At t-CCUS diagnosis, cytogenetics was abnormal in 11 (37%) patients and none had complex karyotype (CK). Paired cytogenetic analysis at t-CCUS and t-MN was available for 10 patients: 4 had unchanged karyotype at t-MN, whereas 6 had cytogenetic evolution. In contrast, 213 (83%) of 256 t-MN patients with available cytogenetics had abnormalities and 126/256 (49%) had CK. Thus, a significantly higher proportion of patients had CK t-MN compared to t-CCUS (χ 2 26.4, P<0.01).
A 42-gene NGS panel identified 36 PV in 21 patients (median 1, range 1-4) at t-CCUS. The most common PV were in TET2 (37.1%), TP53 (11.4%), DNMT3A (8.6%), and ZRSR2 (8.6%) genes. Two patients had paired NGS available at t-CCUS and t-MN: one had BCOR and U2AF1 PV at t-CCUS and acquired an additional NRAS PV at t-MN. The second patient had 2 PV in TP53 at t-CCUS diagnosis, that remained the only PV identified at t-MN. In the t-MN cohort, median number of PV was 2 (range 1-9). The most common PV identified were TP53 (21.5%), TET2 (6.7%), DNMT3A (6.4%), and ASXL1 (6.4%), whereas 4.8% patients had no PV. Three of 22 patients in the t-CCUS cohort had TP53mut, compared to 55 of 157 in the t-MN cohort (χ 2 4, P=0.05).
Management strategies for t-CCUS included observation (20), growth factors (4), treatment of primary malignancy (2), immunosuppressives (1), hypomethylating agent followed by allogeneic SCT (1), and others (2). Following t-CCUS, 9 patients progressed to MDS and 2 to AML with a median of 3.5 months (IQR 1.7-43.4). EFS was 43.4 months (IQR 6.8-80.9). The presence of abnormal cytogenetics (Fig. B) and the absence of DTA-mutations (Fig. C) predicted shorter EFS.
At last follow up, 12 (40%) deaths were noted. Four (33%) patients died without developing t-MN. Causes of death in the t-CCUS cohort were: t-MN (3), primary malignancy (1), progressive cytopenia without MN (1), infection (1), graft-vs.-host-disease (1), and undetermined (5). t-MN patients had a significantly shorter survival compared to t-CCUS (Fig. D).
Conclusion
More than 10% of t-MN patients had a precursor t-CCUS. On the other hand, t-CCUS patients are at a high risk of progression to MN. t-MN is characterized by the acquisition of TP53mut, genomic instability, and a significantly shorter survival compared to t-CCUS. The presence of abnormal cytogenetics and the absence of DTA mutations at t-CCUS predict shorter EFS.
Litzow: Omeros: Other: Advisory Board; AbbVie: Research Funding; Jazz: Other: Advisory Board; Amgen: Research Funding; Astellas: Research Funding; Pluristem: Research Funding; Actinium: Research Funding; Biosight: Other: Data monitoring committee. Patnaik: Kura Oncology: Research Funding; Stemline Therapeutics: Membership on an entity's Board of Directors or advisory committees; Stemline Therapeutics: Membership on an entity's Board of Directors or advisory committees. Al-Kali: Novartis: Research Funding; Astex: Other: Research support to institution.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal